The WiSE-CRT (Wireless Stimulation Endocardially for Cardiac Resynchronization Therapy) system is an innovative, leadless technology designed to treat heart failure patients who are non-responsive to conventional CRT or face challenges with traditional lead placement. Unlike standard CRT devices that require leads to be positioned within the coronary sinus, the WiSE-CRT system delivers left ventricular (LV) pacing endocardially without the need for leads. (1)
The WiSE-CRT device is made up of three components: a conventional pacemaker implant, a leadless pacing device and an ultrasound generator with battery. The ultrasound generator delivers ultrasound to the leadless pacing device, which converts the ultrasound energy into electrical energy to provide endocardial pacing. This provides CRT pacing in patients who may not be able to undergo conventional CRT implantation. (2)
SCST have been made aware of potential interaction between conventional ultrasound, and the WiSE CRT device.
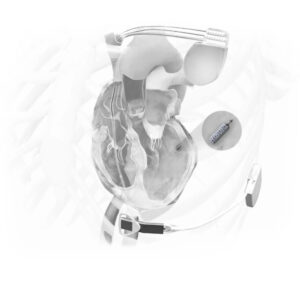
Figure 1: WiSE-CRT device – ebrsystemsinc.com/the-wise-system
The WiSE-CRT device is currently being used experimentally within the U.K. with recent published data. The sites involved are aware of this interaction, however patients may attend other cardiac centres within the U.K. and therefore we feel it is important for SCST to raise awareness of this within our profession.
The manufacturer of the WISE-CRT device, EBR Systems, Inc has provided the following statement:
The precautions section of our IFU states that during TTE, there is a potential for the Receiver Electrode (RE) to convert ultrasonic pulses to stimulation pulses. However, these have been associated with apical or near apical implants of the RE. The precautions also advise that the RE should not be directly targeted. Please note we do not have any patients implanted in the UK with apical implants.
Echocardiography can be performed as per normal in WiSE patients. However, it is important the patient’s heart rhythm is monitored. If additional beats appear to be induced, the echo probe should be removed and the alternate echo protocol should be followed. This can be found at: wiseelabel.com (that hyperlinks to: https://www.ebrsystemsinc.com/wiseelabel)
All sites that follow up WiSE patients have been trained on performing cardiac echo including an alternate echo protocol. Patients also have a specific ID card with warnings and directing sonographers to the alternate echo page.
___________________________________________________
- Sieniewicz BJ, Betts TR, James S et al. Real-world experience of leadless left ventricular endocardial cardiac resynchronization therapy: A multicenter international registry of the WiSE-CRT pacing system. Heart Rhythm 2020;17:1291-1297.
- Singh JP, Rinaldi CA, Sanders P et al. Leadless Ultrasound-Based Cardiac Resynchronization System in Heart Failure. JAMA Cardiology 2024;9:871-879.

Recent Comments